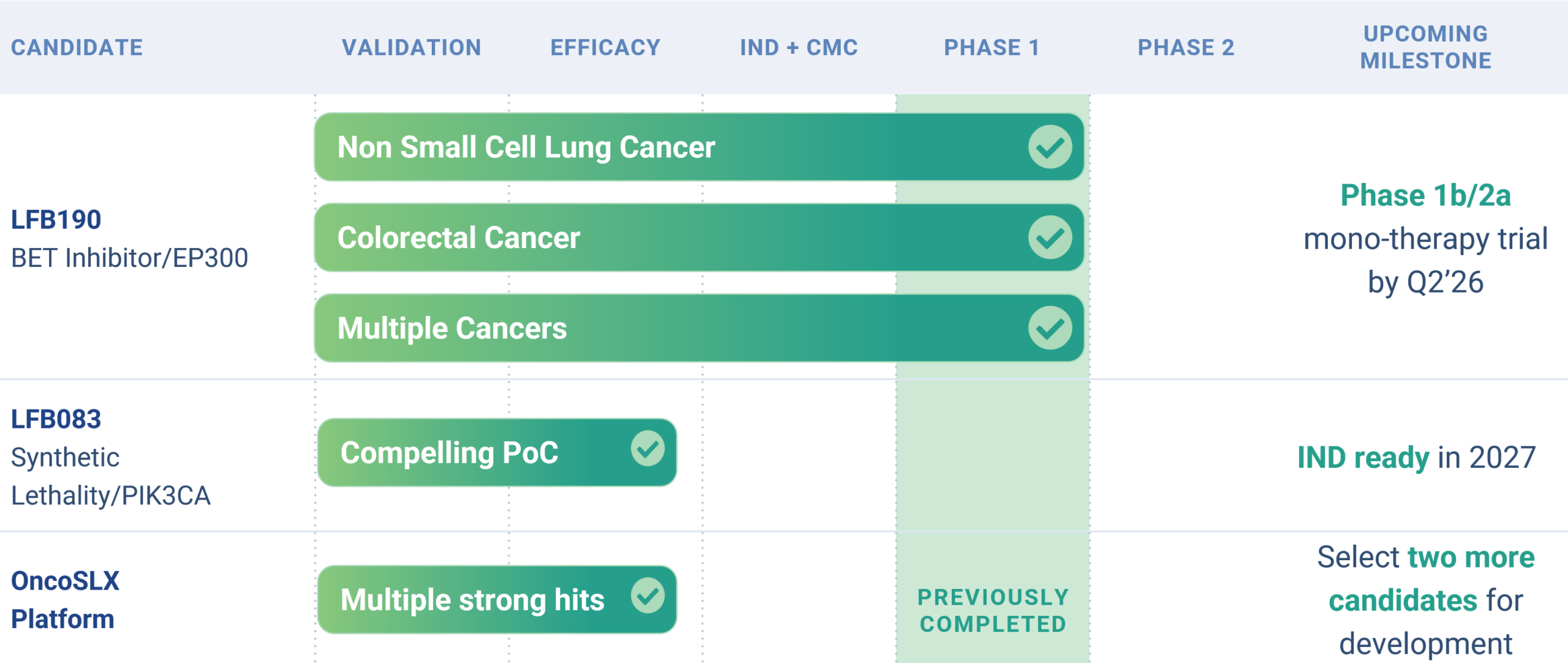
Pipeline
Product Candidates
Leapfrog’s pipeline consists of multiple clinically tested small molecule compounds that have the potential to be blockbuster treatments for genetically defined cancers. Both lead programs have been shown to be safe in approximately 100 unselected patients each.
LFB190
Among the hundreds of pharmacogenetic screens that we have conducted, the sensitizing effect of EP300 loss to BET inhibition is similar to PARP-BRCA1/HRD in magnitude and significance, and among the strongest we have discovered. It holds up in all tumor types where EP300 is a driver, and with all selective BET inhibitors.
Leapfrog Bio is initiating a Phase 1b/2a clinical trial of our lead program, LFB190, in 2026. LFB190 is a best-in-class BET inhibitor being developed for the treatment of EP300 loss-of-function (LOF) mutant cancers, a patient population with significant unmet medical need.
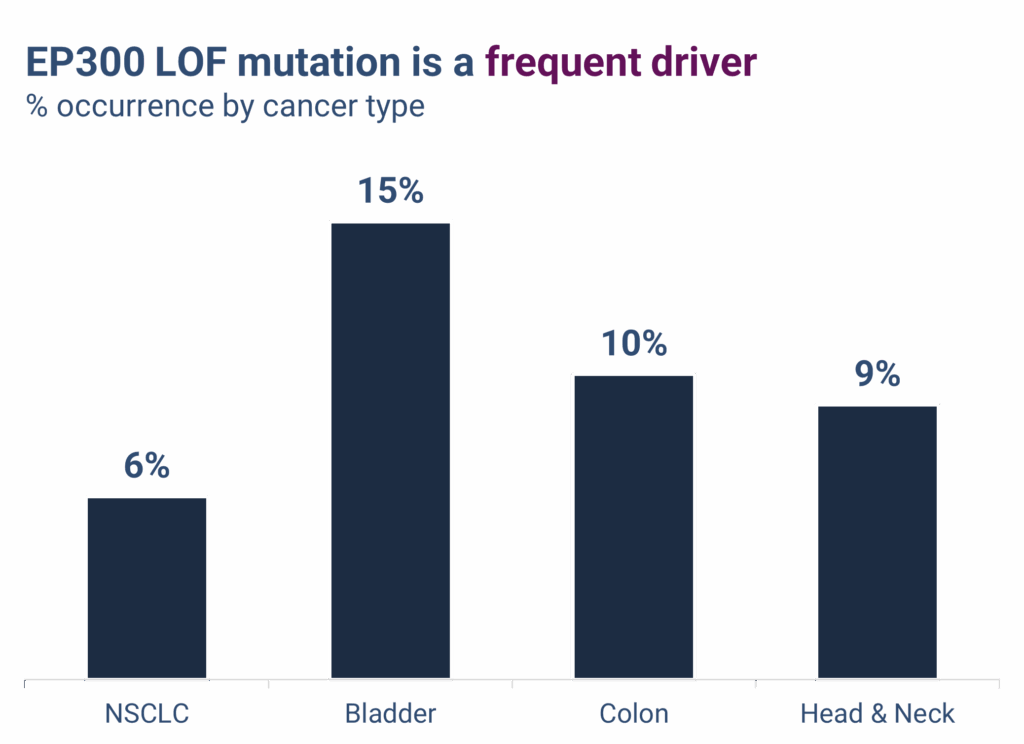
EP300 LOF mutations are well-characterized cancer drivers and occur at meaningful frequencies across multiple solid tumors, including:
- Non-small cell lung cancer (NSCLC): ~6%
- Bladder cancer: ~15%
- Other major solid tumors: 5–10%, including head and neck, esophageal, gastric, and urothelial cancers
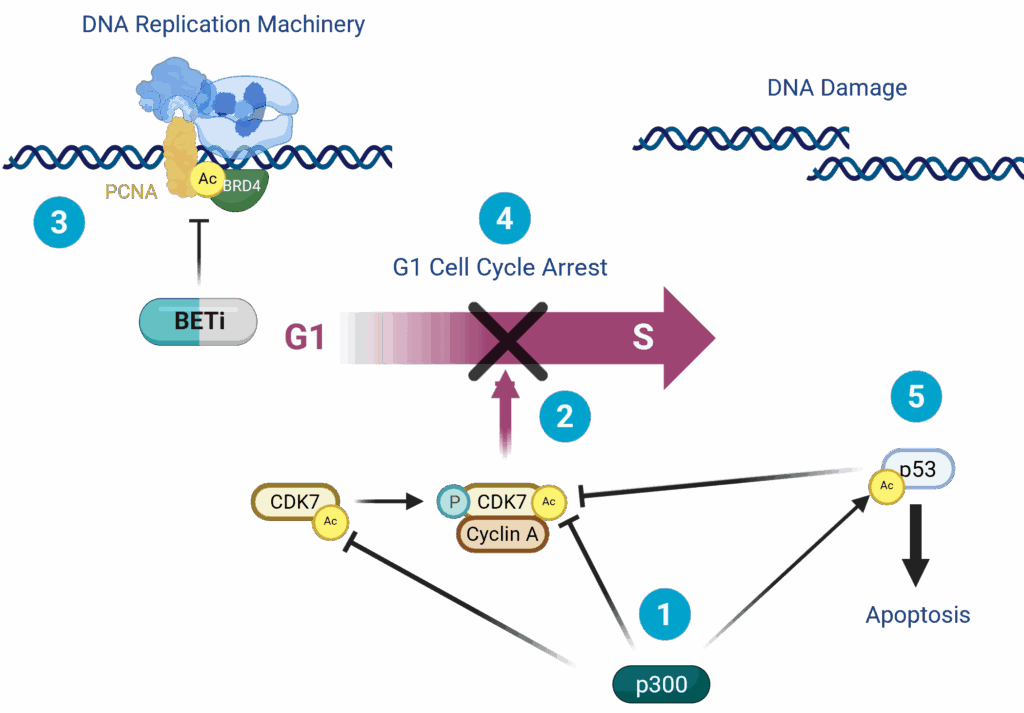
We and others have discovered that BETi disrupts BRD4-dependent recruitment of PCNA and DNA replication machinery, which typically leads to G1 cell cycle arrest. However, in the context of EP300 LOF, cell cycle checkpoint regulation of G1 to S transition is lost and the cells progress anyway, leading to mitotic catastrophe and apoptosis.

LFB083
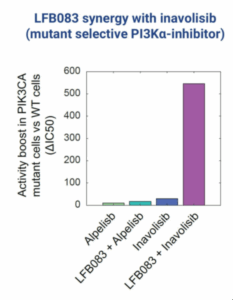
Our second program, LFB083, targets a synthetic lethality relationship with PIK3CA gain of function mutations. These cause more than 100,000 cases of cancer per year in the US alone, including a third of all breast cancer. LFB083 increases activity of mutant selective PI3K alpha inhibitors more than ten-fold with minimal impact on healthy cells.